15358968703

Study on desulfurization technology of blast furnace gas
Blast furnace gas is a by-product gas produced in the process of iron and steel production. Its output is huge, and it can provide fuel for other sections of iron and steel production or be used for power generation. There are many users, but its composition contains sulfur, and the sulfur dioxide in the flue gas after combustion exceeds the emission standard. If desulfuration is carried out in the tail flue gas, it will cause large investment, high operation cost, large floor area, etc., so desulfuration of blast furnace gas is directly carried out, Good economic benefits. At present, the blast furnace gas desulfurization process mainly includes adsorption method and catalytic hydrolysis method, both of which are put into operation, but they also have certain shortcomings.
Keywords blast furnace gas; Carbonyl sulfur; Adsorption method; Catalytic hydrolysis
Blast furnace plays an important role in the long process steel production process. It produces 1600-2000 m3 blast furnace gas per ton of molten iron by-product, with a calorific value of 700 - 800 kcal/m3. The gas contains carbon monoxide, carbon dioxide, nitrogen, hydrogen and other components, accompanied by dust, sulfur and other pollutants. In general, blast furnace gas is used in hot blast furnace, sintering, heating furnace, power generation and other processes after gravity and bag dedusting, and finally discharged into the atmosphere. The emission concentration of sulfur dioxide in the flue gas is 100-200 mg/m3.
In may2018, the Ministry of ecological environment issued the work plan for ultra low emission transformation of iron and steel enterprises (Draft for comments). In November 2019, Shandong Province's emission standard of air pollutants for iron and steel industry db37/990-2019 was officially implemented. Iron and steel enterprises officially need to build or transform desulfurization and denitration facilities, hot blast furnaces, heating furnaces See Table 1 for S02 emission requirements in the combustion tail gas of blast furnace gas user units such as gas power generation.
However, for the blast furnace gas user unit currently in use, the concentration of S02 in the tail gas after combustion cannot meet the requirements specified in the new standard.

The technical route for sulfur emission control of blast furnace gas mainly includes front-end control, i.e. removing sulfur from blast furnace gas, and terminal control of blast furnace gas combustion, and setting up desulfurization and denitration devices at the user end. The front-end control is adopted for centralized treatment before use by the user, and the sulfur content in the gas is controlled at the concentration that does not exceed the standard after combustion. In this way, the device is centralized, and the investment is low compared with the end control. If the terminal control is adopted, it is necessary to install desulfurization facilities in the blast furnace gas section, which will cause high investment, high operation cost and large land occupation.
Blast furnace gas desulfurization is a key transformation project of iron and steel in the next few years. As most of the sulfides in blast furnace gas are carbonyl sulfur, carbon disulfide and other organic sulfur, it is impossible to directly learn from coke oven gas desulfurization and other technologies at present.
Sulfur composition in 1 blast furnace gas
Smelting molten iron in blast furnace is to use coke, coal and iron ore to produce reduction reaction. The sulfur in blast furnace gas is mainly in reduced state. The calorific value gas of blast furnace gas is mainly C0 (20%~25%), H2 (1%), which contains a small amount of sulfide. After chromatographic sampling and detection, the sulfide in blast furnace gas mainly includes hydrogen sulfide, carbonyl sulfur, carbon disulfide, etc.
The sulfide distribution in blast furnace gas is shown in Table 2.

It can be seen from table 2 that organic sulfur accounts for the largest proportion of sulfide in blast furnace gas, accounting for about 70%-80%, and inorganic sulfur accounts for 20%-30%.
Carbonyl sulfur is also called carbon oxysulfide or carbonyl sulfur. Its molecular formula is cos or OCS. Its molecular structure is simple and belongs to linear molecular structure. Oxygen, carbon and sulfur atoms are connected by double bonds. Its molecular structure is compact and approximate to ellipsoidal type, so its chemical properties are relatively stable. Carbonyl sulfur is weakly acidic. Compared with H2S and CS2, it is not easy to dissociate and liquefy and difficult to remove [1].
2 blast furnace gas desulfurization technology
2.1 catalytic hydrolysis
The hydrolysis catalyst is generally composed of A12O3, TiO2 or their mixture, and impregnated with a certain amount of alkali metal, alkaline earth metal or transition metal. Weak alkalinity is the active center of COS hydrolysis reaction, and the reaction rate is related to the alkalinity of catalyst. Generally, the stronger the alkalinity is, the faster the reaction rate is. However, SO2, CO2, O2 and steam will compete with cos on the active center of catalyst and affect the hydrolysis activity of COS; S02 and 02 can also promote irreversible sulfation of the catalyst and poison the catalyst [2-3].
Fe3O4 can also be used as the hydrolysis catalyst to catalyze the hydrolysis of COS and CS2 to produce H2S and C02. The hydrolyzed H2S is absorbed by alkaline substances such as NaOH. See (formula 1) and (formula 2) for the comprehensive reaction formula:
Cos+h2o → h2s+co2 (equation 1)
H2s+naoh → na2s+h2o (formula 2)
It can be seen from the above reaction formula that this process route will produce waste water, so attention should be paid to the destination and treatment process of this part of waste water. In addition, the catalytic hydrolysis tower is generally located in front of the TRT, and its pressure drop needs to be controlled to reduce the impact on the TRT efficiency.
See Figure 1 for process flow.

2.2 adsorption method
The adsorption method is currently used in the desulfurization of coke oven gas. The gas is adsorbed by the adsorption material, and the separation effect is achieved by using the characteristics of the adsorption material and the great specific surface area. Adsorption materials mainly include activated carbon, molecular sieve, zinc oxide, etc.
Activated carbon is made from lignite, coconut shell and wood. It has a large microporous structure and a very high specific surface area, which is also the reason for its desulfurization. In addition, activated carbon is easy to obtain raw materials, low cost, reusable, and waste activated carbon can be used as fuel without solid waste. It is more and more used as desulfurizer [4]. Activated carbon is mostly used in flue gas or other organic gases with low sulfur concentration. Its specific surface area is large and there is residual adsorption on surface particles [5-6].
Bagreev a et al. [7] studied the adsorption capacity of activated carbon impregnated with potassium hydroxide, amines and oxides for COS and H2S, and found that the desulfurization effect was greatly improved compared with that before impregnation. Jiajianguo [8] of Tianjin University impregnated coconut shell activated carbon with potassium carbonate, sodium carbonate, potassium hydroxide and a series of different impregnation concentrations. Finally, it was found that the coconut shell activated carbon impregnated with 6% potassium carbonate solution had the best desulfurization effect, which was 30% higher than that before impregnation. Bandosz et al. [9] studied the effect of activated carbon on H? As for the removal of H2S, they found that the removal of H2S by activated carbon is related to the pH of the adsorption point. When the pH value at the adsorption point is less than 5, it will inhibit the dissociation of H2S and reduce the adsorption capacity of activated carbon for H2S. When the pH value at the adsorption point is more than 5, it will lead to the generation of high concentration of hydrogen sulfide ions, which will oxidize it to polysulfide, thereby increasing the adsorption effect [6].
Zinc oxide desulfurizer has high desulfurization efficiency, large sulfur capacity, stable reaction process and relatively simple process. The sulfur content of flue gas adsorbed by zinc oxide desulfurizer can be as low as 10-9 (volume fraction) [10]. In addition, zinc oxide is often used as desulfurizer for post-treatment of reduction method and hydrolysis method. Zinc oxide can react with cos as follows.
Zno+cos → zns+co2 (equation 3)
See Figure 2 for process flow.
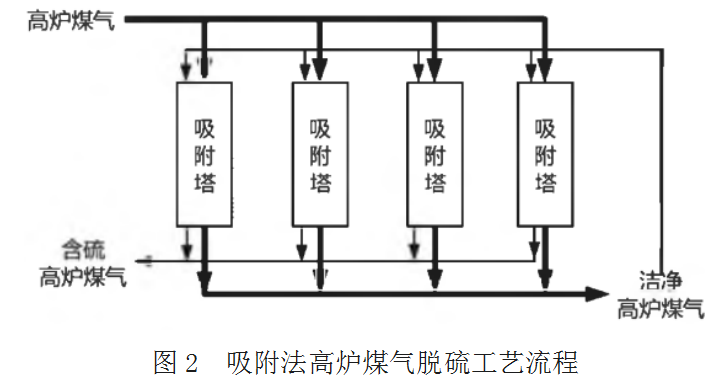
The process flow is to use activated carbon or other adsorption materials to adsorb the organic sulfur in blast furnace gas, and then desorb it through clean gas back blowing. The desorbed sulfur-containing gas is sent to the section equipped with desulfurization and denitration devices, such as sintering, gas power generation, heating furnace, etc. the investment of this process flow is high, so it must be considered that the high sulfur gas is laid separately to the user.
3 conclusion
(1) The front-end fine desulfurization of blast furnace gas can greatly reduce the investment and operation cost of desulfurization and denitration for subsequent users.
(2) The hydrolysis desulfurization process has low investment and small floor area, but the catalyst replacement cycle is short, which will produce desulfurization wastewater. Sodium hydroxide is used as desulfurizer. The content of carbon dioxide in blast furnace gas is 15%~20%, and the content of sulfur is 100-200 mg/m3. Carbon dioxide and hydrogen sulfide will react with sodium hydroxide solution. The generated sodium carbonate and sodium sulfide enter the wastewater, and the wastewater treatment cost shall be considered.
(3) The adsorption desulfurization process has high investment, low operation cost, no waste water, high desulfurization accuracy, and the total sulfur content is less than 500 mg/m3. It is more suitable for the adsorption method. It is insensitive to the fluctuation of sulfur content in the gas, but it does not completely solve the problem of organic sulfur, and only concentrates it to the next user.
(4) Users should select their own process according to the actual production conditions and the advantages and disadvantages of the process.